Warning: Invalid argument supplied for foreach() in /home/starvict/abscience.com.tw/wp-content/themes/wp-abs/single-product.php on line 28
 ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit
ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit
L00350
Brand
Warning: Invalid argument supplied for foreach() in /home/starvict/abscience.com.tw/wp-content/themes/wp-abs/single-product.php on line 76
Description
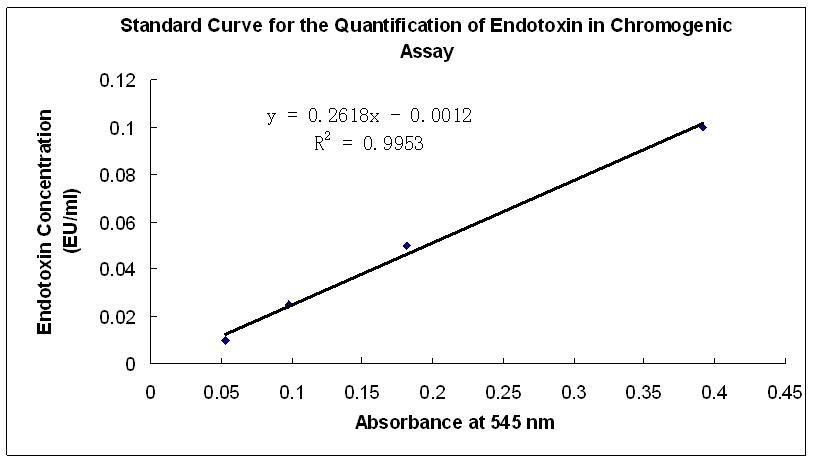
Genscript ToxinSensor™ Chromogenic LAL Endotoxin Assay Kit is designed to be a quantitative In Vitro end-point endotoxin test for human and animal parenteral drugs, biological products, and medical devices.The system is not intended for detection of endotoxin in a licensed reagent, clinical samples or the diagnosis of human disease. A measurable endotoxin concentration range from 0.01 to 1 EU/ml can be achieved. Lyophilized Amebocyte Lysate reagent is made from amebocyte lysate from the horseshoe crab (Tachypleus tridentatus).
Key Features :
Highly Sensitive: Detect endotoxin concentration in the range of 0.01 – 1 EU/ml
Fast: The incubation period can be shortened to 14 minutes
Reliable: Color-stabilizer ensures accurate results
Ready-to-use: Kit includes endotoxin-free tips and tubes, LAL reagent water and incubation rack
Broad application: Quantitative in vitro end-point endotoxin test
Application
Reactivity